What Does Kj Stand for in Food
Phytocannabinoid discovered in 1940
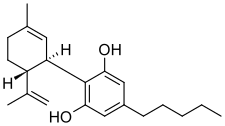 | |
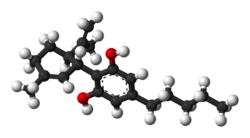 | |
| Clinical data | |
|---|---|
| Trade names | Epidiolex, Epidyolex |
| Other names | CBD, cannabidiolum, (−)-cannabidiol[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618051 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Inhalation (smoking, vaping), buccal (aerosol spray),[3] [4] oral (solution)[5] [6] |
| Drug class | Cannabinoid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | • Oral: 6% (fasted)[11] • Inhaled: 31% (11–45%)[12] |
| Elimination half-life | 18–32 hours[13] |
| Identifiers | |
| IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| PDB ligand |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.215.986 |
| Chemical and physical data | |
| Formula | C 21 H 30 O 2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) |
|
| Melting point | 66 °C (151 °F) |
| Solubility in water | insoluble |
| SMILES
| |
| InChI
| |
| (verify) | |
Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract.[14] As of 2019, clinical research on CBD included studies related to anxiety, cognition, movement disorders, and pain, but there is insufficient high-quality evidence that cannabidiol is effective for these conditions.[15] [16]
Cannabidiol can be taken internally in multiple ways, including by inhaling cannabis smoke or vapor, by mouth, and as an aerosol spray into the cheek.[3] [4] It may be supplied as CBD oil containing only CBD as the active ingredient (excluding tetrahydrocannabinol [THC] or terpenes), CBD-dominant hemp extract oil, capsules,[17] dried cannabis, or prescription liquid solution.[6] [16] CBD does not have the same psychoactivity as THC,[18] [19] and may change the effects of THC on the body if both are present.[14] [18] [20] [21] As of 2018[update], the mechanism of action for its biological effects has not been determined.[18] [20]
In the United States, the cannabidiol drug Epidiolex was approved by the Food and Drug Administration in 2018 for the treatment of two epilepsy disorders.[22] While the 2018 United States Farm Bill removed hemp and hemp extracts (including CBD) from the Controlled Substances Act, the marketing and sale of CBD formulations for medical use or as an ingredient in dietary supplements or manufactured foods remains illegal under FDA regulation, as of 2021.[23] [24]
Medical uses [edit]
Cannabidiol is the generic name of the drug and its INN.[25]
Research [edit]
As of 2019[update], there was limited high-quality evidence for cannabidiol having a neurological effect in humans.[15] [16] [26] [27]
Epilepsy [edit]
In the United States, the FDA has indicated only one brand of prescription cannabidiol called "Epidiolex" for the treatment of seizures associated with Dravet syndrome, Lennox-Gastaut syndrome, or tuberous sclerosis complex in people one year of age and older.[28] [6] [22] [29] [30] While Epidiolex treatment is generally well tolerated, it is associated with minor adverse effects, such as gastrointestinal upset, decreased appetite, lethargy, sleepiness and poor sleep quality.[22] [29] [30]
In the European Union, cannabidiol (Epidyolex) is indicated for use as adjunctive therapy of seizures associated with Lennox Gastaut syndrome (LGS) or Dravet syndrome (DS), in conjunction with clobazam, for people two years of age and older.[10] In 2020, the label for Epidiolex in the US was expanded to include seizures associated with tuberous sclerosis complex. Epidiolex/Epidyolex is the first prescription formulation of plant-derived cannabidiol approved by regulatory bodies in the US and Europe.[31]
Other uses [edit]
Research on other uses for cannabidiol includes several neurological disorders, but the findings have not been confirmed to establish such uses in clinical practice.[13] [15] [16] [18] [32] [33] [26] In October 2019, the FDA issued an advisory warning that the effects of CBD during pregnancy or breastfeeding are unknown, indicating that the safety, doses, interactions with other drugs or foods, and side effects of CBD are not clinically defined, and may pose a risk to the mother and infant.[34]
Many claims are made for the therapeutic benefit of cannabidiol that are not backed by sound evidence. Some claims, such as treatment of cancer, are pseudoscience.[35]
In 2020, the label for Epidiolex in the US was expanded to include treatment of seizures associated with tuberous sclerosis complex.[28]
Non-intoxicating effects [edit]
Cannabidiol does not appear to have any intoxicating effects[36] (i.e., "getting high") such as those caused by ∆9-THC in Cannabis, but it is under preliminary research for its possible anti-anxiety and anti-psychotic effects.[15] [16] [19] As the legal landscape and understanding about the differences in medical cannabinoids unfolds, experts are working to distinguish "medical cannabis" (with varying degrees of psychotropic effects and deficits in executive function) from "medical CBD therapies", which would commonly present as having a reduced or non-psychoactive side-effect profile.[16] [19] [37]
Various strains of "medical cannabis" are found to have a significant variation in the ratios of CBD-to-THC and are known to contain other non-psychotropic cannabinoids.[38] Any psychoactive cannabis, regardless of its CBD content, is derived from the flower (or bud) of the genus Cannabis. As defined by US federal law, non-psychoactive hemp (also commonly termed "industrial hemp"), regardless of its CBD content, is any part of the cannabis plant, whether growing or not, containing a ∆9-tetrahydrocannabinol concentration of no more than 0.3% on a dry-weight basis.[39] Certain standards are required for legal growing, cultivating, and producing the hemp plant, but there are no federal standards for quality being enforced in the hemp industry. Certain state regulations are in place, but vary state to state.[40] For instance, the Colorado Industrial Hemp Program registers growers of industrial hemp and samples crops to verify that the dry-weight THC concentration does not exceed 0.3%.[39]
Side effects [edit]
Research indicates that cannabidiol may reduce adverse effects of THC, particularly those causing intoxication and sedation, but only at high doses.[41] Safety studies of cannabidiol showed it is well tolerated, but may cause tiredness, diarrhea, or changes in appetite as common adverse effects.[42] Epidiolex documentation lists sleepiness, insomnia and poor quality sleep, decreased appetite, diarrhea, and fatigue.[6] [43]
Potential interactions [edit]
Laboratory evidence indicated that cannabidiol may reduce THC clearance, increasing plasma concentrations which may raise THC availability to receptors and enhance its effect in a dose-dependent manner.[44] [45] In vitro, cannabidiol inhibited the activity of voltage-dependent sodium and potassium channels, which may affect neural activity.[46] A recent study using X-ray crystallography showed that CBD binds inside the sodium channel pore at a novel site at the interface of the fenestrations and the central hydrophobic cavity of the channel. Binding at this site blocks the transmembrane-spanning sodium ion translocation pathway, providing a molecular mechanism for channel inhibition, which could contribute to a reduced excitability.[47] A small clinical trial reported that CBD partially inhibited the CYP2C-catalyzed hydroxylation of THC to 11-OH-THC.[48] Little is known about potential drug interactions, but CBD mediates a decrease in clobazam metabolism.[49]
Veterinary medicine [edit]
Research [edit]
The number of research projects and scientific publications on cannabidiol and other cannabinoids in pets has surged in the late 2010s.
As of December 2020, there are no hemp-derived, cannabinoid-rich registered veterinary medicinal products in any of the major regions (see Legal status across countries).
In the USA and other territories there are, however, numerous veterinary nutraceutical products available OTC. The lack of clarity in the regulations governing veterinary hemp food supplements allows for products of questionable quality to flood the market,[50] [51] which may pose a risk to the wellbeing of pets and owners.
To understand better the benefits of CBD and associated compounds for the quality of life of animals, companies specialized in CBD products for animals have been funding research projects.[52] [53] [54] [55] [56] [57] [58] [59] [60] [61]
Canine osteoarthritis [edit]
CBD's ability to help regulate the endocannabinoid system[62] [63] [64] and reduce the release of excitatory neurotransmitters could result in a retrograde inhibitory signal that lessens chronic pain responses. Studies in dogs suffering from chronic pain associated with osteoarthritis showed an increase in level of activity in animals receiving CBD-rich food supplements.[60] [53] [57] [55]
Epilepsy [edit]
From the results seen in humans with drugs such as Epidiolex and Sativex in scientific studies and reviews,[65] it could be expected that CBD-based products would be helpful to manage seizures in dogs. However, despite the numerous case reports presented by veterinary neurologists supporting the benefits of CBD as adjunctive therapy, as of December 2020, published controlled studies have not shown a statistically significant decrease in the number of seizures across the groups receiving CBD.[52] [56] Further research in this area is required before any clear conclusion can be drawn.
Pharmacokinetics [edit]
The oral bioavailability of CBD varies greatly across species and it is linked to the presentation and the time of administration.[61] [58] [66] A 24-hour kinetic examination in dogs showed that the absorption of the cannabidiolic acid (CBDA) does occur, and that this molecule is absorbed least twice as well as CBD post oral ingestion.[61] [58] [67]
It was found that the major metabolites of CBD in humans (7-OH-CBD and 7-COOH-CBD) are not prevalent in dogs, while 6-OH-CBD was found to be the primary metabolite in dogs receiving a CBD-enriched cannabis-derived herbal extract,[68] suggesting that canine and human CBD metabolic route might be somewhat different.[66]
Safety [edit]
Increases in Alkaline Phosphatase (ALP) have been reported in various canine studies.[60] [61] [57] A 39-week study in dogs evaluating doses of 0, 10, 50, and 100 mg of CBD/kg reported an increase of up to 8x in the ALP in the 100 mg/kg group, and of up to 1.5x in Alanine Aminotransferase (ALT) in the 50 mg/kg and 100 mg/kg groups. However, there were no clinical signs associated with these results.[69]
Pharmacology [edit]
Pharmacodynamics [edit]
Cannabidiol has low affinity for the cannabinoid CB1 and CB2 receptors,[70] [71] although it can act as an antagonist of CB1/CB2 agonists despite this low affinity.[71] Cannabidiol may be an antagonist of GPR55, a G protein-coupled receptor and putative cannabinoid receptor that is expressed in the caudate nucleus and putamen in the brain.[72] [73] It also may act as an inverse agonist of GPR3, GPR6, and GPR12.[74] CBD has been shown to act as a serotonin 5-HT1A receptor partial agonist.[75] At higher concentrations, CBD acts as an inverse agonist of 5-HT1A receptors.[76] It is an allosteric modulator of the μ- and δ-opioid receptors as well.[77] The pharmacological effects of CBD may involve PPARγ agonism, inhibition of voltage-gated cation channels, and intracellular calcium release.[14]
Pharmacokinetics [edit]
The oral bioavailability of cannabidiol is approximately 6% in humans, while its bioavailability via inhalation is 11 to 45% (mean 31%).[11] [12] The elimination half-life of CBD is 18–32 hours.[13] Cannabidiol is metabolized in the liver as well as in the intestines by the cytochrome P450 enzymes CYP2B6, CYP2C19, CYP2D6, CYP2J2, and CYP3A4, and by the isoenzymes UGT1A7, UGT1A9, and UGT2B7,[78] [79] [80] [6] forming a variety of metabolites such as 7-hydroxycannabidiol as well as the 6α- and 6β-hydroxy isomers and derivatives hydroxylated on the alkyl side chain, followed by glucuronidation.[81] CYP3A4 facilitates decarbonylation of CBD to liberate carbon monoxide, a bioactive gasotransmitter and pharmaceutical candidate.[82] CBD may have a wide margin in dosing.[33]
Pharmaceutical preparations [edit]
Nabiximols (brand name Sativex), an oromucosal spray made of a complex botanical mixture containing cannabidiol (CBD), delta-9-tetrahydrocannabinol (THC), and additional cannabinoid and non-cannabinoid constituents from cannabis sativa plants, was approved by Health Canada in 2005 to treat central neuropathic pain in multiple sclerosis, and in 2007 for cancer-related pain.[83] In New Zealand, Sativex is "approved for use as an add-on treatment for symptom improvement in people with moderate to severe spasticity due to multiple sclerosis who have not responded adequately to other anti-spasticity medication."[84]
Epidiolex is an orally administered cannabidiol solution. It was approved in 2018 by the US Food and Drug Administration (FDA) for treatment of two rare forms of childhood epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,[22] and seizures associated with tuberous sclerosis complex.[28] In the US, it is approved in these indications for patients one year of age and older.
Chemistry [edit]
At room temperature, cannabidiol is a colorless crystalline solid.[85] In strongly basic media and the presence of air, it is oxidized to a quinone.[86] Under acidic conditions it cyclizes to THC,[87] which also occurs during pyrolysis,[88] but not during combustion (smoking).[89] The synthesis of cannabidiol has been accomplished by several research groups.[90] [91] [92]
-

Possible mechanism of intramolecular cyclization of CBD to Δ9-THC[93]
Biosynthesis [edit]

Cannabidiol and THC biosynthesis[94]
Cannabis produces CBD-carboxylic acid through the same metabolic pathway as THC, until the next to last step, where CBDA synthase performs catalysis instead of THCA synthase.[95]
Isomerism [edit]

| Formal numbering | Terpenoid numbering | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | Structure | |||
|---|---|---|---|---|---|---|---|---|
| Short name | Chiral centers | Full name | Short name | Chiral centers | ||||
| Δ5-Cannabidiol | 1 and 3 | 2-(6-isopropenyl-3-methyl-5-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ4-Cannabidiol | 1 and 3 | 4 | No | Unscheduled | 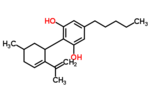 |
| Δ4-Cannabidiol | 1, 3 and 6 | 2-(6-isopropenyl-3-methyl-4-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ5-Cannabidiol | 1, 3 and 4 | 8 | No | Unscheduled |  |
| Δ3-Cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-3-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ6-Cannabidiol | 3 and 4 | 4 | ? | Unscheduled |  |
| Δ3,7-Cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methylenecyclohex-1-yl)-5-pentyl-1,3-benzenediol | Δ1,7-Cannabidiol | 3 and 4 | 4 | No | Unscheduled | 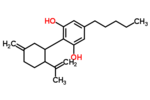 |
| Δ2-Cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ1-Cannabidiol | 3 and 4 | 4 | Yes | Unscheduled |  |
| Δ1-Cannabidiol | 3 and 6 | 2-(6-isopropenyl-3-methyl-1-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ2-Cannabidiol | 1 and 4 | 4 | No | Unscheduled | 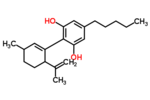 |
| Δ6-Cannabidiol | 3 | 2-(6-isopropenyl-3-methyl-6-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ3-Cannabidiol | 1 | 2 | No | Unscheduled | 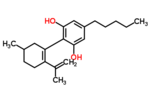 |
Pyrolysis [edit]
In the typical operating temperature range of e-cigarettes (250–400 °C), 25–52% of CBD is transformed into other chemical substances: Δ9-THC, Δ8-THC, cannabinol and cannabichromene as predominant pyrolysates. From a chemical point of view, CBD in e-cigarettes can be considered as a precursor of THC.[96]
History [edit]
Efforts to isolate the active ingredients in cannabis were made in the 19th century.[97] Cannabidiol was studied in 1940 from Minnesota wild hemp[97] and Egyptian Cannabis indica resin.[98] [99] The chemical formula of CBD was proposed from a method for isolating it from wild hemp.[97] Its structure and stereochemistry were determined in 1963.[100]
Plant breeding [edit]
Selective breeding of cannabis plants has expanded and diversified as commercial and therapeutic markets develop.[101] Some growers in the US succeeded in lowering the proportion of CBD-to-THC to accommodate customers who preferred varietals that were more mind-altering due to the higher THC and lower CBD content.[102] In the US, hemp is classified by the federal government as cannabis containing no more than 0.3% THC by dry weight. This classification was established in the 2018 Farm Bill and was refined to include hemp-sourced extracts, cannabinoids, and derivatives in the definition of hemp.[103]
Society and culture [edit]
Foods and beverages [edit]

An example of beverages claiming to contain CBD in a Los Angeles grocery
Food and beverage products containing cannabidiol were widely marketed in the United States as early as 2017.[104] Hemp seed ingredients which do not naturally contain THC or CBD (but which may be contaminated with trace amounts on the outside during harvesting) were declared by the US Food and Drug Administration (FDA) as Generally recognized as safe (GRAS) in December 2018. CBD itself has not been declared GRAS, and under US federal law is illegal to sell as a food, dietary supplement, or animal feed.[101] State laws vary considerably as non-medical cannabis and derived products have been legalized in some jurisdictions in the 2010s.
Similar to energy drinks and protein bars which may contain vitamin or herbal additives, food and beverage items can be infused with CBD as an alternative means of ingesting the substance. In the United States, numerous products are marketed as containing CBD, but in reality contain little or none.[101] [105] Some companies marketing CBD-infused food products with claims that are similar to the effects of prescription drugs have received warning letters from the Food and Drug Administration for making unsubstantiated health claims.[101] [106] In February 2019, the New York City Department of Health announced plans to fine restaurants that sell food or drinks containing CBD, beginning in October 2019.[107]
Sports [edit]
Cannabidiol has been used by professional and amateur athletes across disciplines and countries, with the World Anti-Doping Agency removing CBD from its banned substances list. The United States Anti-Doping Agency and United Kingdom-Anti-Doping Agency do not have anti-CBD policies, with the latter stating that, "CBD is not currently listed on the World Anti-Doping Agency Prohibited List. As a result, it is permitted to use in sport, though the intended benefits are unclear and not backed by clinical evidence.. All other cannabinoids (including but not limited to cannabis, hashish, marijuana, and THC) are prohibited in-competition. The intention of the regulations is to prohibit cannabinoids that activate the same receptors in the brain as activated by THC."[108] [109] In 2019, the cannabis manufacturer, Canopy Growth, acquired majority ownership of BioSteel Sports Nutrition, which is developing CBD products under endorsement by numerous professional athletes.[110] The National Hockey League Alumni Association began a project with Canopy Growth to determine if CBD or other cannabis products might improve neurological symptoms and quality of life in head-injured players.[110] Numerous professional athletes use CBD, primarily for treating pain.[110] [111] [112]
Legal status across countries [edit]
Australia [edit]
Prescription medicine (Schedule 4) for therapeutic use containing two percent (2.0%) or less of other cannabinoids commonly found in cannabis (such as ∆9-THC). A Schedule 4 drug under the SUSMP is a Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by State or Territory legislation to prescribe and should be available from a pharmacist on prescription.[113]
In June 2020, the Australian Therapeutic Goods Administration (TGA) published a consultation on a proposal to pave the way to make "low dose" CBD available to consumer/patients via pharmacists only through moving products from Schedule 4 to 3.[114] Any products sold would need to have their safety, quality and efficacy pre-assessed by the TGA and be formally approved for sale (details to be outlined by TGA). They would be made available to over 18s only, with the maximum daily dose of 60 mg/day, up to 2% THC finished product allowed, 30-day maximum supply, plant-derived or synthetic. This proposal is based on an initial literature review on the safety of low dose CBD published by the TGA in April 2020.[115] Epidyolex was approved, for the adjunctive therapy of seizures associated with Lennox-Gastaut syndrome or with Dravet syndrome, on 18 September 2020, and added to the ARTG on 21 September 2020.[2] [116]
Bulgaria [edit]
In 2020, Bulgaria became the first country in the European Union to allow retail sales of food products and supplements containing CBD, despite the ongoing discussion within the EU about the classification of CBD as a novel food.[117] But there exists a legal gap because of the lack of a legally-permissible minimum amount of THC in the products containing cannabinoids.[118]
Canada [edit]
In October 2018, cannabidiol became legal for recreational and medical use by the federal Cannabis Act.[119] [120] [121] As of August 2019, CBD products in Canada could only be sold by authorized retailers or federally licensed medical companies, limiting their access to the general public.[122] The Canadian government states that CBD products "are subject to all of the rules and requirements that apply to cannabis under the Cannabis Act and its regulations."[119] It requires "a processing licence to manufacture products containing CBD for sale, no matter what the source of the CBD is, and that CBD and products containing CBD, such as cannabis oil, may only be sold by an authorized retailer or licensed seller of medical CBD."[119] Edible CBD products were scheduled to be permitted for sale in Canada on October 17, 2019 for human consumption.[119]
As of August 2020, it is still illegal to carry cannabis and cannabis-derived products (including products containing CBD) across the Canadian border. If one carries any amount of cannabis for any purpose (including medical), it needs to be declared to the Canada Border Services Agency. Not declaring it is a serious criminal offence.[123]
European Union [edit]

A CBD product vending machine in Italy
In 2019, the European Commission announced that CBD and other cannabinoids would be classified as "novel foods",[124] meaning that CBD products would require authorization under the EU Novel Food Regulation stating that because "this product was not used as a food or food ingredient before May 15, 1997, before it may be placed on the market in the EU as a food or food ingredient, a safety assessment under the Novel Food Regulation is required."[125] The recommendation – applying to CBD extracts, synthesized CBD, and all CBD products, including CBD oil – was scheduled for a final ruling by the European Commission in March 2019.[124] If approved, manufacturers of CBD products would be required to conduct safety tests and prove safe consumption, indicating that CBD products would not be eligible for legal commerce until at least 2021.[124] In December 2020, the European Commission concluded that CBD should not be considered as drug and can be qualified as food.[126]
Cannabidiol is listed in the EU Cosmetics Ingredient Database (CosIng).[127] However, the listing of an ingredient, assigned with an INCI name, in CosIng does not mean it is to be used in cosmetic products or is approved for such use.[127]
Several industrial hemp varieties can be legally cultivated in Western Europe. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1%, with THC less than 0.3%.[128]
New Zealand [edit]
In 2017, the government made changes to the regulations so that restrictions would be removed, which meant a doctor was able to prescribe cannabidiol to patients.[129]
The passing of the Misuse of Drugs (Medicinal Cannabis) Amendment Act in December 2018 means cannabidiol is no longer a controlled drug in New Zealand, but is a prescription medicine under the Medicines Act, with the restriction that "the tetrahydrocannabinols (THCs) and specified substances within the product must not exceed 2 percent of the total CBD, tetrahydrocannabinol (THC) and other specified substances.".[130]
Russian Federation [edit]
According to a document received in response to an appeal to the Ministry of Internal Affairs of the Russian Federation, measures of state control in the Russian Federation regarding CBD have not been established. However, there is also a response from the Ministry of Health of the Russian Federation indicating that CBD can be considered as an isomer of restricted THC. But in this case, the THC isomer is also, for example, progesterone, which is freely sold in pharmacies.[131] On February 17, 2020, the deputy of the Moscow City Duma Darya Besedina sent an official request to the Prime Minister of the Russian Federation Mikhail Mishustin with a request to eliminate that legal ambiguity by publishing official explanations and, if necessary, making required changes in the corresponding government decree.[132]
Sweden [edit]
Cannabidiol is classified as a medical product in Sweden.[133] However, in July 2019, Supreme Court of Sweden ruled that CBD oil with any concentration of THC falls under the narcotic control laws.[134]
Switzerland [edit]
While THC remains illegal, cannabidiol is not subject to the Swiss Narcotic Acts because this substance does not produce a comparable psychoactive effect.[135] Cannabis products containing less than 1% THC can be sold and purchased legally.[136] [137]
Ukraine [edit]
On 7 April 2021 the Ukrainian government legalised use of isolated cannabidiol. Additionally, it approved Nabiximols, a cannabidiol-containing drug, for medical use.[138]
United Kingdom [edit]
Cannabidiol, in an oral-mucosal spray formulation combined with Delta-9-tetrahydrocannabinol, is a product available (by prescription only prior to 2017) for the relief of severe spasticity due to multiple sclerosis (where other anti-spasmodics have not been effective).[139]
Until 2017, products containing cannabidiol marketed for medical purposes were classed as medicines by the UK regulatory body, the Medicines and Healthcare products Regulatory Agency (MHRA), and could not be marketed without regulatory approval for the medical claims.[140] [141] As of 2018[update], cannabis oil is legal to possess, buy, and sell in the UK, providing the product does not contain more than 1 milligram of THC and is not advertised as providing a medicinal benefit.[142]
In January 2019, the UK Food Standards Agency indicated it would regard CBD products, including CBD oil, as a novel food having no history of use before May 1997, and stated that such products must have authorisation and proven safety before being marketed.[124] [143] The deadline for companies with existing products to submit a full and validated novel foods application with the FSA is 31 March 2021; failure to do so before this date will exclude those companies from selling CBD.[144] New products containing CBD after this deadline will require a fully approved application.[145]
United Nations [edit]
Cannabidiol is not scheduled under the Convention on Psychotropic Substances or any other UN drug treaties.[146] In 2018, the World Health Organization recommended that CBD remain unscheduled.[147] [148]
United States [edit]
As of 2021[update], cannabidiol extracted from marijuana remains a Schedule I Controlled Substance,[101] [149] [150] and is not approved as a prescription drug or dietary supplement or allowed for interstate commerce in the United States.[151] CBD derived from hemp (with 0.3% THC or lower) is legal to sell as a cosmetics ingredient or for other purposes not regulated by the FDA, but cannot be sold under federal law as an ingredient in food, dietary supplement, or animal feed.[101] [152] It is a common misconception that the legal ability to sell hemp (which may contain CBD), and hemp extracts and derivatives (including CBD), makes CBD legal for sale as a supplement or medicine.[152] [153]
In September 2018, following its approval by the FDA for rare types of childhood epilepsy,[22] the GW Pharmaceuticals drug Epidiolex was rescheduled (by the Drug Enforcement Administration) as a Schedule V drug to allow for its prescription use.[154] Epidiolex still requires rescheduling in some states before it can be prescribed in those states.[155] [156]
In 2013, a CNN program that featured Charlotte's Web cannabis brought increased attention to the use of CBD in the treatment of seizure disorders.[157] [158] Since then, 16 states have passed laws to allow the use of CBD products with a physician's recommendation (instead of a prescription) for treatment of certain medical conditions.[159] This is in addition to the 30 states that have passed comprehensive medical cannabis laws, which allow for the use of cannabis products with no restrictions on THC content.[159] Of these 30 states, eight have legalized the use and sale of cannabis products without requirement for a physician's recommendation.[159] As of October 2020, CBD was not an FDA-approved drug eligible for interstate commerce,[101] and the FDA encouraged manufacturers to follow procedures for drug approval.[160]
Federal illegality has made it difficult historically to conduct research on CBD.[161] Cannabidiol is currently the subject of an FDA investigational new drug evaluation, and is not considered legal as a dietary supplement or food ingredient, as of October 2020[update].[101] [162] CBD is openly sold in head shops and health food stores in some states where such sales have not been explicitly legalized.[163]
State and local governments may also regulate cannabidiol. For example, the Massachusetts Department of Agricultural Resources issued a rule in June 2019 aligning state CBD regulations with FDA regulations. This means that although recreational marijuana is legal in the state, CBD cannot legally be sold in food or as a dietary supplement under state law.[164]
Health concerns [edit]
In November 2019, the FDA issued concerns about the safety of cannabidiol, stating that CBD use has potential to cause liver injury, interfere with the mechanisms of prescription drugs, produce gastrointestinal disorders, or affect alertness and mood.[151] In October 2020, the FDA updated its safety concerns about CBD,[101] acknowledging the unknown effects of protracted use, how it affects the developing brain, fetus or infants during breastfeeding, whether it interacts with dietary supplements or prescription drugs, whether male fertility is affected, and its possible side effects, such as drowsiness.[165]
In February 2020, the UK FSA advised vulnerable people, such as pregnant women, breastfeeding mothers, and those already taking medication for other medical concerns not to take CBD. The FSA further recommended that healthy adults should not consume more than 70 mg CBD per day.[144]
Mislabeling and poisoning [edit]
Studies conducted by the FDA from 2014 through 2019 have determined that a majority of CBD products are not accurately labeled with the amount of CBD they contain.[166] For example, a 2017 analysis of cannabidiol content in oil, tincture, or liquid vape products purchased online in the United States showed that 69% were mislabeled, with 43% having higher and 26% having lower content than stated on product labels.[167] [168]
In 2020, the FDA conducted a study of 147 CBD products and found that half contained THC.[166] [169]
As of September 2019[update], 1,085 people contacted US poison control centers about CBD-induced illnesses, doubling the number of cases over the 2018 rate and increasing by 9 times the case numbers of 2017.[170] Of cases reported in 2019, more than 33% received medical attention and 46 people were admitted to a hospital intensive care unit, possibly due to exposure to other products, or drug interactions with CBD.[168]
2018 Farm Bill and hemp [edit]
The 2014 Farm Bill[171] legalized the sale of "non-viable hemp material" grown within states participating in the Hemp Pilot Program which defined hemp as cannabis containing less than 0.3% of THC.[172] The 2018 United States Farm Bill removed the hemp plant and all "derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis," including CBD, from the Controlled Substances Act, making them legal to manufacture in the United States.[23] [173] [174] The FDA retains regulatory authority over hemp-derived CBD,[153] while the DEA is not involved in the regulation of legally-compliant hemp and hemp products.[175] The 2018 Farm Bill requires that research and development of CBD for a therapeutic purpose would have to be conducted under notification and reporting to the FDA.[153] [165] In the United States, hemp-derived CBD is legal to sell for industrial purposes or as a cosmetics ingredient, but under FDA regulation it cannot be marketed for medical use or as an ingredient in food, dietary supplements, or animal feed.[101]
FDA warning letters [edit]
From 2015 to December 2020, the FDA issued dozens of warning letters to American manufacturers of CBD products for false advertising and illegal interstate marketing of CBD as an unapproved drug to treat diseases, such as cancer, osteoarthritis, symptoms of opioid withdrawal, Alzheimer's disease, and pet disorders.[50] [176] The FDA said that the letters were issued to enforce action against companies that were deceiving consumers by marketing illegal products for which there was insufficient evidence of safety and efficacy to treat diseases.[50] In July 2019, the FDA stated: "Selling unapproved products with unsubstantiated therapeutic claims — such as claims that CBD products can treat serious diseases and conditions — can put patients and consumers at risk by leading them to put off important medical care. Additionally, there are many unanswered questions about the science, safety, effectiveness and quality of unapproved products containing CBD."[50]
In December 2020, the Federal Trade Commission (FTC) initiated a law enforcement crackdown on American companies marketing CBD products as unapproved drugs.[177] [178] The warning also applied to hemp CBD capsules and oil that were being marketed illegally while not adhering to the federal definition of a dietary supplement.[178]
See also [edit]
- Hash oil
- Hemp oil
- List of investigational antipsychotics
- List of investigational analgesics
References [edit]
- ^ "cannabidiol (CHEBI:69478)". www.ebi.ac.uk.
- ^ a b "Epidyolex". Therapeutic Goods Administration (TGA). September 29, 2020. Retrieved September 30, 2020.
- ^ a b Itin C, Barasch D, Domb AJ, Hoffman A (May 2020). "Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol". International Journal of Pharmaceutics. 581: 119276. doi:10.1016/j.ijpharm.2020.119276. PMID 32243971. S2CID 214785913.
- ^ a b Itin C, Domb AJ, Hoffman A (October 2019). "A meta-opinion: cannabinoids delivered to oral mucosa by a spray for systemic absorption are rather ingested into gastro-intestinal tract: the influences of fed / fasting states". Expert Opinion on Drug Delivery. 16 (10): 1031–1035. doi:10.1080/17425247.2019.1653852. PMID 31393180. S2CID 199505274.
- ^ "Sativex (Cannabidiol/Tetrahydrocannabinol) Bayer Label" (PDF). bayer.ca . Retrieved June 28, 2018.
- ^ a b c d e f "Epidiolex- cannabidiol solution". DailyMed. August 26, 2020. Retrieved September 11, 2020.
- ^ "Epidyolex 100 mg/ml oral solution - Summary of Product Characteristics (SmPC)". (emc). August 28, 2020. Retrieved September 11, 2020.
- ^ "Sativex Oromucosal Spray - Summary of Product Characteristics (SmPC)". (emc). August 25, 2020. Retrieved September 11, 2020.
- ^ "LeafPro CBDmed Oil FS QP 20%- cannabidiol oil". DailyMed. March 9, 2020. Retrieved September 11, 2020.
- ^ a b "Epidyolex EPAR". European Medicines Agency (EMA). June 24, 2019. Retrieved September 11, 2020. Text was copied from this source which is copyrighted by the European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b Perucca E, Bialer M (June 5, 2020). "Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications". CNS Drugs. 34 (8): 795–800. doi:10.1007/s40263-020-00741-5. PMID 32504461. S2CID 219313952.
- ^ a b Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G (May 2009). "Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders". Phytotherapy Research (Review). 23 (5): 597–602. doi:10.1002/ptr.2625. PMID 18844286. S2CID 21836765.
- ^ a b c Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. (June 2014). "Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders". Epilepsia. 55 (6): 791–802. doi:10.1111/epi.12631. PMC4707667. PMID 24854329.
- ^ a b c Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS (December 2012). "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences (Review). 367 (1607): 3364–78. doi:10.1098/rstb.2011.0389. PMC3481531. PMID 23108553.
- ^ a b c d Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. (December 2019). "Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis". The Lancet. Psychiatry. 6 (12): 995–1010. doi:10.1016/S2215-0366(19)30401-8. PMC6949116. PMID 31672337.
- ^ a b c d e f VanDolah HJ, Bauer BA, Mauck KF (September 2019). "Clinicians' Guide to Cannabidiol and Hemp Oils". Mayo Clinic Proceedings. 94 (9): 1840–1851. doi:10.1016/j.mayocp.2019.01.003. PMID 31447137.
- ^ "What Are CBD Capsules?". CBD Oil King. May 14, 2019. Retrieved October 21, 2019.
- ^ a b c d Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. (July 2017). "Cannabidiol: State of the art and new challenges for therapeutic applications". Pharmacology & Therapeutics. 175: 133–150. doi:10.1016/j.pharmthera.2017.02.041. PMID 28232276.
- ^ a b c Iseger TA, Bossong MG (March 2015). "A systematic review of the antipsychotic properties of cannabidiol in humans". Schizophrenia Research. 162 (1–3): 153–61. doi:10.1016/j.schres.2015.01.033. PMID 25667194. S2CID 3745655.
- ^ a b Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M (January 2018). "Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ9-Tetrahydrocannabinol". Neuropsychopharmacology. 43 (1): 142–154. doi:10.1038/npp.2017.209. PMC5719112. PMID 28875990.
- ^ Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–31. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ^ a b c d e "FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy". US Food and Drug Administration (FDA). June 25, 2018. Retrieved June 25, 2018.
- ^ a b Mead A (June 14, 2019). "Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States". Frontiers in Plant Science. 10: 697. doi:10.3389/fpls.2019.00697. PMC6590107. PMID 31263468.
- ^ "FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). #2. How does the 2018 Farm Bill define hemp? What does it mean for FDA-regulated products?". US Food and Drug Administration (FDA). January 22, 2021. Retrieved April 14, 2021.
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO Drug Information. 30 (2): 241. 2016.
- ^ a b Prud'homme M, Cata R, Jutras-Aswad D (2015). "Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence". Substance Abuse. 9: 33–8. doi:10.4137/SART.S25081. PMC4444130. PMID 26056464.
- ^ Hoch E, Niemann D, von Keller R, Schneider M, Friemel CM, Preuss UW, et al. (February 2019). "How effective and safe is medical cannabis as a treatment of mental disorders? A systematic review". European Archives of Psychiatry and Clinical Neuroscience. 269 (1): 87–105. doi:10.1007/s00406-019-00984-4. PMC6595000. PMID 30706168.
- ^ a b c Office of the Commissioner (July 31, 2020). "FDA Approves New Indication for Drug Containing an Active Ingredient Derived from Cannabis to Treat Seizures in Rare Genetic Disease". FDA . Retrieved November 25, 2020.
- ^ a b Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, et al. (July 2018). "Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence". Journal of Neurology, Neurosurgery, and Psychiatry. 89 (7): 741–753. doi:10.1136/jnnp-2017-317168. PMID 29511052.
- ^ a b Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, et al. (February 2020). "Cannabis-based products for pediatric epilepsy: An updated systematic review". Seizure. 75: 18–22. doi:10.1016/j.seizure.2019.12.006. PMID 31865133. S2CID 208878465.
- ^ Commissioner, Office of the (March 27, 2020). "FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy". FDA . Retrieved May 28, 2021.
- ^ Silva TB, Balbino CQ, Weiber AF (May 2015). "The relationship between cannabidiol and psychosis: A review". Annals of Clinical Psychiatry. 27 (2): 134–41. PMID 25954940.
- ^ a b Blessing EM, Steenkamp MM, Manzanares J, Marmar CR (October 2015). "Cannabidiol as a Potential Treatment for Anxiety Disorders". Neurotherapeutics. 12 (4): 825–36. doi:10.1007/s13311-015-0387-1. PMC4604171. PMID 26341731.
- ^ "What You Should Know About Using Cannabis, Including CBD, When Pregnant or Breastfeeding". US Food and Drug Administration (FDA). October 16, 2019. Retrieved October 17, 2019.
- ^ Novella S (September 30, 2020). "Where Are We With CBD?". Science-Based Medicine.
- ^ Kicman A, Toczek M (September 2020). "The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease". International Journal of Molecular Sciences. 21 (18): 6740. doi:10.3390/ijms21186740. PMC7554803. PMID 32937917.
- ^ Sachs J, McGlade E, Yurgelun-Todd D (October 2015). "Safety and Toxicology of Cannabinoids". Neurotherapeutics. 12 (4): 735–46. doi:10.1007/s13311-015-0380-8. PMC4604177. PMID 26269228.
- ^ Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (October 2009). "Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb". Trends in Pharmacological Sciences. 30 (10): 515–27. doi:10.1016/j.tips.2009.07.006. PMID 19729208.
- ^ a b "Industrial hemp". Department of Agriculture, State of Colorado. 2018. Retrieved September 14, 2018.
- ^ "Cannabinoid Clinical | Cannabinoids Research, Effects, and Uses". CannabinoidClinical.com . Retrieved October 27, 2020.
- ^ Fischer B, Russell C, Sabioni P, van den Brink W, Le Foll B, Hall W, et al. (August 2017). "Lower-Risk Cannabis Use Guidelines: A Comprehensive Update of Evidence and Recommendations". American Journal of Public Health. 107 (8): e1–e12. doi:10.2105/AJPH.2017.303818. PMC5508136. PMID 28644037.
- ^ Iffland K, Grotenhermen F (2017). "An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies". Cannabis and Cannabinoid Research. 2 (1): 139–154. doi:10.1089/can.2016.0034. PMC5569602. PMID 28861514.
- ^ "Cannabidiol (CBD): MedlinePlus Supplements". medlineplus.gov . Retrieved January 15, 2021.
- ^ Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ (August 1995). "Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain". Drug Metabolism and Disposition. 23 (8): 825–31. PMID 7493549.
- ^ Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, et al. (November 2011). "Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats". Psychopharmacology. 218 (2): 443–57. doi:10.1007/s00213-011-2342-0. PMID 21667074. S2CID 6240926.
- ^ Ghovanloo MR, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ (October 2018). "Inhibitory effects of cannabidiol on voltage-dependent sodium currents". The Journal of Biological Chemistry. 293 (43): 16546–16558. doi:10.1074/jbc.RA118.004929. PMC6204917. PMID 30219789.
- ^ Sait LG, Sula A, Ghovanloo MR, Hollingworth D, Ruben PC, Wallace BA (October 2020). "Cannabidiol interactions with voltage-gated sodium channels". eLife. 9. doi:10.7554/eLife.58593. PMC7641581. PMID 33089780.
- ^ Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM (December 2005). "Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract". Therapeutic Drug Monitoring. 27 (6): 799–810. doi:10.1097/01.ftd.0000177223.19294.5c. PMID 16306858. S2CID 12979224.
- ^ Lucas CJ, Galettis P, Schneider J (November 2018). "The pharmacokinetics and the pharmacodynamics of cannabinoids". British Journal of Clinical Pharmacology. 84 (11): 2477–2482. doi:10.1111/bcp.13710. PMC6177698. PMID 30001569.
- ^ a b c d "FDA warns company marketing unapproved cannabidiol products with unsubstantiated claims to treat cancer, Alzheimer's disease, opioid withdrawal, pain and pet anxiety". US Food and Drug Administration (FDA). July 23, 2019. Retrieved July 24, 2019.
Unlike drugs approved by the FDA, the manufacturing process of these products has not been subject to FDA review as part of the drug approval process, and there has been no FDA evaluation of whether these products are effective for their intended use, what the proper dosage is, how they could interact with FDA-approved drugs, or whether they have dangerous side effects or other safety concerns.
- ^ Wakshlag JJ, Cital S, Eaton SJ, Prussin R, Hudalla C (April 15, 2020). "Cannabinoid, Terpene, and Heavy Metal Analysis of 29 Over-the-Counter Commercial Veterinary Hemp Supplements". Veterinary Medicine: Research and Reports. 11: 45–55. doi:10.2147/vmrr.s248712. PMC7169471. PMID 32346530.
- ^ a b McGrath S, Bartner LR, Rao S, Packer RA, Gustafson DL (June 2019). "Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy". Journal of the American Veterinary Medical Association. 254 (11): 1301–1308. doi:10.2460/javma.254.11.1301. PMID 31067185. S2CID 148569810.
- ^ a b Brioschi FA, Di Cesare F, Gioeni D, Rabbogliatti V, Ferrari F, D'Urso ES, et al. (August 2020). "Oral Transmucosal Cannabidiol Oil Formulation as Part of a Multimodal Analgesic Regimen: Effects on Pain Relief and Quality of Life Improvement in Dogs Affected by Spontaneous Osteoarthritis". Animals. 10 (9): 1505. doi:10.3390/ani10091505. PMC7552307. PMID 32858828.
- ^ Morris EM, Kitts-Morgan SE, Spangler DM, McLeod KR, Costa JH, Harmon DL (September 22, 2020). "The Impact of Feeding Cannabidiol (CBD) Containing Treats on Canine Response to a Noise-Induced Fear Response Test". Frontiers in Veterinary Science. 7: 569565. doi:10.3389/fvets.2020.569565. PMC7537661. PMID 33195551.
- ^ a b Verrico CD, Wesson S, Konduri V, Hofferek CJ, Vazquez-Perez J, Blair E, et al. (September 2020). "A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain". Pain. 161 (9): 2191–2202. doi:10.1097/j.pain.0000000000001896. PMC7584779. PMID 32345916.
- ^ a b Mogi C, Fukuyama T (2019). "Cannabidiol as a potential anti-epileptic dietary supplement in dogs with suspected epilepsy: three case reports". Pet Behaviour Science (7): 11–16. doi:10.21071/pbs.v0i7. ISSN 2445-2874.
- ^ a b c Kogan L, Hellyer P, Downing R (2020). "Hemp Oil Extract to Treat Canine Osteoarthritis-Related Pain: A Pilot Study". Journal of the American Holistic Veterinary Medical Association. 58: 35–45.
- ^ a b c Wakshlag JJ, Schwark WS, Deabold KA, Talsma BN, Cital S, Lyubimov A, et al. (September 4, 2020). "Pharmacokinetics of Cannabidiol, Cannabidiolic Acid, Δ9-Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Related Metabolites in Canine Serum After Dosing With Three Oral Forms of Hemp Extract". Frontiers in Veterinary Science. 7: 505. doi:10.3389/fvets.2020.00505. PMC7498943. PMID 33102539.
- ^ Hannon MB, Deabold KA, Talsma BN, Lyubimov A, Iqbal A, Zakharov A, et al. (September 2020). "Serum cannabidiol, tetrahydrocannabinol (THC), and their native acid derivatives after transdermal application of a low-THC Cannabis sativa extract in beagles". Journal of Veterinary Pharmacology and Therapeutics. 43 (5): 508–511. doi:10.1111/jvp.12896. PMID 32735381. S2CID 220889224.
- ^ a b c Gamble LJ, Boesch JM, Frye CW, Schwark WS, Mann S, Wolfe L, et al. (July 23, 2018). "Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs". Frontiers in Veterinary Science. 5: 165. doi:10.3389/fvets.2018.00165. PMC6065210. PMID 30083539.
- ^ a b c d Deabold KA, Schwark WS, Wolf L, Wakshlag JJ (October 2019). "Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats". Animals. 9 (10): 832. doi:10.3390/ani9100832. PMC6826847. PMID 31635105.
- ^ Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. (September 2003). "Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS". Proceedings of the National Academy of Sciences of the United States of America. 100 (18): 10529–33. Bibcode:2003PNAS..10010529I. doi:10.1073/pnas.1834309100. PMC193595. PMID 12917492.
- ^ Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A (February 2004). "Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin". The Journal of Pharmacology and Experimental Therapeutics. 308 (2): 446–53. doi:10.1124/jpet.103.060079. PMID 14610224. S2CID 17663780.
- ^ Jung KM, Piomelli D (2015). "Cannabinoids and Endocannabinoids". In Pfaff DW, Volkow ND (eds.). Neuroscience in the 21st Century. New York, NY: Springer New York. pp. 1–31. doi:10.1007/978-1-4614-6434-1_136-1. ISBN978-1-4614-6434-1.
- ^ Russo E (2001). Handbook of psychotropic herbs : a scientific analysis of herbal remedies for psychiatric conditions. New York: Haworth Herbal Press. ISBN0-7890-0718-5. OCLC 43810871.
- ^ a b Vaughn D, Kulpa J, Paulionis L (February 11, 2020). "Preliminary Investigation of the Safety of Escalating Cannabinoid Doses in Healthy Dogs". Frontiers in Veterinary Science. 7: 51. doi:10.3389/fvets.2020.00051. PMC7029731. PMID 32118071.
- ^ Della Rocca G, Di Salvo A (July 28, 2020). "Hemp in Veterinary Medicine: From Feed to Drug". Frontiers in Veterinary Science. 7: 387. doi:10.3389/fvets.2020.00387. PMC7399642. PMID 32850997.
- ^ Chicoine A, Illing K, Vuong S, Pinto KR, Alcorn J, Cosford K (September 29, 2020). "Pharmacokinetic and Safety Evaluation of Various Oral Doses of a Novel 1:20 THC:CBD Cannabis Herbal Extract in Dogs". Frontiers in Veterinary Science. 7: 583404. doi:10.3389/fvets.2020.583404. PMC7550466. PMID 33134364.
- ^ "Drug Approval Package: Epidiolex (Cannabidiol)" (PDF). U.S. FDA. June 14, 2018. pp. 30–31.
- ^ Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO (August 2007). "Cannabidiol--recent advances". Chemistry & Biodiversity (Review). 4 (8): 1678–92. doi:10.1002/cbdv.200790147. PMID 17712814. S2CID 3689072.
- ^ a b Pertwee RG (January 2008). "The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin". British Journal of Pharmacology. 153 (2): 199–215. doi:10.1038/sj.bjp.0707442. PMC2219532. PMID 17828291.
- ^ Gray RA, Whalley BJ (January 2020). "The proposed mechanisms of action of CBD in epilepsy". Epileptic Disorders. 22 (S1): 10–15. doi:10.1684/epd.2020.1135 (inactive October 31, 2021). PMID 32053110. CS1 maint: DOI inactive as of October 2021 (link)
- ^
- ^ Laun AS, Shrader SH, Brown KJ, Song ZH (March 2019). "GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol". Acta Pharmacologica Sinica. 40 (3): 300–308. doi:10.1038/s41401-018-0031-9. PMC6460361. PMID 29941868.
- ^ Russo EB, Burnett A, Hall B, Parker KK (August 2005). "Agonistic properties of cannabidiol at 5-HT1a receptors". Neurochemical Research. 30 (8): 1037–43. doi:10.1007/s11064-005-6978-1. PMID 16258853. S2CID 207222631.
- ^ Martínez-Aguirre C, et al. (December 2020). "Cannabidiol Acts at 5-HT1A Receptors in the Human Brain: Relevance for Treating Temporal Lobe Epilepsy". Front. Behav. Neurosci. 14: 611278. doi:10.3389/fnbeh.2020.611278. PMC7770178. PMID 33384591.
- ^ Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E (February 2006). "Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 372 (5): 354–61. doi:10.1007/s00210-006-0033-x. PMID 16489449. S2CID 4877869.
- ^ Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K (2013). "Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19" (PDF). Drug Metabolism and Pharmacokinetics. 28 (4): 332–8. doi:10.2133/dmpk.dmpk-12-rg-129. PMID 23318708. S2CID 4884324. Archived from the original (PDF) on February 8, 2020.
- ^ Qian Y, Gurley BJ, Markowitz JS (2019). "The Potential for Pharmacokinetic Interactions Between Cannabis Products and Conventional Medications". Journal of Clinical Psychopharmacology. 39 (5): 462–471. doi:10.1097/JCP.0000000000001089. PMID 31433338. S2CID 201118659.
- ^ Yamaori S, Okamoto Y, Yamamoto I, Watanabe K (November 2011). "Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6". Drug Metabolism and Disposition. 39 (11): 2049–56. doi:10.1124/dmd.111.041384. PMID 21821735. S2CID 20558596.
- ^ Vlad RA, Hancu G, Ciurba A, Antonoaea P, Rédai EM, Todoran N, Silasi O, Muntean DL (October 2020). "Cannabidiol - therapeutic and legal aspects". Die Pharmazie. 75 (10): 463–469. doi:10.1691/ph.2020.0076 (inactive October 31, 2021). PMID 33305718. CS1 maint: DOI inactive as of October 2021 (link)
- ^ Hopper CP, Zambrana PN, Goebel U, Wollborn J (June 2021). "A brief history of carbon monoxide and its therapeutic origins". Nitric Oxide. 111–112: 45–63. doi:10.1016/j.niox.2021.04.001. PMID 33838343. S2CID 233205099.
- ^ Russo EB (February 2008). "Cannabinoids in the management of difficult to treat pain". Therapeutics and Clinical Risk Management. 4 (1): 245–59. doi:10.2147/TCRM.S1928. PMC2503660. PMID 18728714.
- ^ "Sativex Oromucosal Spray". Medsafe, New Zealand Medicines and Medical Devices Safety Authority. December 19, 2018. Retrieved April 3, 2019.
- ^ Jones PG, Falvello L, Kennard O, Sheldrick GM, Mechoulam R (1977). "Cannabidiol". Acta Crystallogr. B. 33 (10): 3211–3214. doi:10.1107/S0567740877010577.
- ^ Mechoulam R, Ben-Zvi Z, Gaoni Y (August 1968). "Hashish--13. On the nature of the Beam test". Tetrahedron. 24 (16): 5615–24. doi:10.1016/0040-4020(68)88159-1. PMID 5732891.
- ^ Gaoni Y, Mechoulam R (1966). "Hashish—VII The isomerization of cannabidiol to tetrahydrocannabinols". Tetrahedron. 22 (4): 1481–1488. doi:10.1016/S0040-4020(01)99446-3.
- ^ Küppers FJ, Bercht CA, Salemink CA, Lousberg RC, Terlouw JK, Heerma W (1975). "Cannabis—XV: Pyrolysis of cannabidiol. Structure elucidation of four pyrolytic products". Tetrahedron. 31 (13–14): 1513–1516. doi:10.1016/0040-4020(75)87002-5.
- ^ Quarles W, Ellman G, Jones R (1973). "Toxicology of marijuana: conditions for conversion of cannabidiol to THC upon smoking". Clinical Toxicology. 6 (2): 211–6. doi:10.3109/15563657308990520. PMID 4715204.
- ^ Petrzilka T, Haefliger W, Sikemeier C, Ohloff G, Eschenmoser A (March 1967). "[Synthesis and optical rotation of the (-)-cannabidiols]". Helvetica Chimica Acta. 50 (2): 719–23. doi:10.1002/hlca.19670500235. PMID 5587099.
- ^ Gaoni Y, Mechoulam R (1985). "Boron trifluoride etherate on alumuna — a modified Lewis acid reagent. An improved synthesis of cannabidiol". Tetrahedron Letters. 26 (8): 1083–1086. doi:10.1016/S0040-4039(00)98518-6.
- ^ Kobayashi Y, Takeuchi A, Wang YG (June 2006). "Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate". Organic Letters. 8 (13): 2699–702. doi:10.1021/ol060692h. PMID 16774235.
- ^ Gaoni Y, Mechoulam R (January 1966). "Hashish—VII: The isomerization of cannabidiol to tetrahydrocannabinols". Tetrahedron. 22 (4): 1481–8. doi:10.1016/S0040-4020(01)99446-3.
- ^ Taura F, Sirikantaramas S, Shoyama Y, Yoshikai K, Shoyama Y, Morimoto S (June 2007). "Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa". FEBS Letters. 581 (16): 2929–34. doi:10.1016/j.febslet.2007.05.043. PMID 17544411. S2CID 20253070.
- ^ Marks MD, Tian L, Wenger JP, Omburo SN, Soto-Fuentes W, He J, et al. (2009). "Identification of candidate genes affecting Delta9-tetrahydrocannabinol biosynthesis in Cannabis sativa". Journal of Experimental Botany. 60 (13): 3715–26. doi:10.1093/jxb/erp210. PMC2736886. PMID 19581347.
- ^ Czégény Z, Nagy G, Babinszki B, Bajtel Á, Sebestyén Z, Kiss T, et al. (April 2021). "CBD, a precursor of THC in e-cigarettes". Scientific Reports. 11 (1): 8951. Bibcode:2021NatSR..11.8951C. doi:10.1038/s41598-021-88389-z. PMC8076212. PMID 33903673.
- ^ a b c Adams R, Hunt M, Clark JH (1940). "Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp". Journal of the American Chemical Society. 62 (1): 196–200. doi:10.1021/ja01858a058. ISSN 0002-7863.
- ^ Jacob A, Todd AR (1940). "Cannabidiol and cannabol, constituents of Cannabis indica resin". Nature. 145 (3670): 350. Bibcode:1940Natur.145..350J. doi:10.1038/145350a0. ISSN 0028-0836. S2CID 4106662.
- ^ Work TS, Bergel F, Todd AR (January 1939). "The active principles of Cannabis indica resin. I". The Biochemical Journal. 33 (1): 123–7. doi:10.1042/bj0330123. PMC1264344. PMID 16746878.
- ^ Mechoulam R, Shvo Y (December 1963). "Hashish. I. The structure of cannabidiol". Tetrahedron. 19 (12): 2073–8. doi:10.1016/0040-4020(63)85022-x. PMID 5879214.
- ^ a b c d e f g h i j "FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD) (General)". US Food and Drug Administration (FDA). January 22, 2021. Retrieved April 14, 2021.
- ^ Romney L (September 13, 2012). "On the frontier of medical pot to treat boy's epilepsy". Los Angeles Times.
- ^ "7 U.S. Code § 5940 – Legitimacy of industrial hemp research". LII / Legal Information Institute . Retrieved November 27, 2018.
- ^ "Billboard featuring hemp leaf raises questions about new beverage for sale in Cincinnati | WLWT5". WLWT5. September 29, 2017. Retrieved September 29, 2017.
- ^ "Warning Letters and Test Results for Cannabidiol-Related Products". US Food and Drug Administration (FDA). November 2, 2017. Retrieved January 2, 2018.
- ^ Fox A, Ravitz JR, Leongini EM, Malkin BJ (November 20, 2017). "Companies Marketing CBD Products Be Warned: FDA Is Watching". Lexology . Retrieved December 14, 2017.
- ^ La Vito A, Franck T (February 15, 2019). "New York City plans to fine restaurants using CBD in food and drinks". CNBC . Retrieved February 19, 2019.
- ^ "Athlete Advisory Note: Cannabidiol (CBD)". United Kingdom Anti-Doping Agency. Retrieved October 9, 2019.
- ^ "Athletes: 6 things to know about cannabidiol". US Anti-Doping Agency. October 23, 2018. Retrieved February 17, 2020.
- ^ a b c Donna S (October 2, 2019). "Canopy cannabis company buys ex-NHL player's sports nutrition business". CTV Business. The Canadian Press. Retrieved February 17, 2020.
BioSteel's brand ambassadors also include well-known athletes across major sports leagues in North America, which could be beneficial as the company's attempt to push regulated CBD nutrition products into the mainstream health and wellness segments
- ^ Loudin A (December 7, 2019). "As more pro athletes use cannabis for aches and pain, the more they run afoul of rules". The Washington Post . Retrieved February 17, 2020.
- ^ Harrison S (September 28, 2019). "Lots of athletes say CBD Is a better painkiller. Is It?". Wired . Retrieved February 17, 2020.
- ^ "Poisons Standard June 2017". Legislation.gov.au. Retrieved December 4, 2016.
- ^ Australian Government Department of Health Therapeutic Goods Administration (April 24, 2020). "Consultation: Proposed amendments to the Poisons Standard - Joint ACMS/ACCS meetings, June 2020". Therapeutic Goods Administration (TGA) . Retrieved November 25, 2020.
- ^ "Safety of low dose cannabidiol" (PDF). Government of Australia. Therapeutic Goods Administration. April 2020.
- ^ "Summary for ARTG Entry: 328860 Epidyolex cannabidiol 100 mg/mL oral liquid solution bottle" (PDF). Therapeutic Goods Administration (TGA). Government of Australia. Retrieved September 30, 2020.
- ^ Hasse J. "This EU country has become the first to allow free sale Of CBD". Forbes . Retrieved February 21, 2020.
- ^ Todorova S (July 29, 2019). "Growing cannabis in Bulgaria: Legal but still stigmatised". Lexology . Retrieved September 3, 2020.
- ^ a b c d "Cannabidiol (CBD)". Government of Canada. August 13, 2019. Retrieved October 11, 2019.
- ^ "Health products containing cannabis or for use with cannabis: Guidance for the Cannabis Act, the Food and Drugs Act, and related regulations". Government of Canada. July 11, 2018. Retrieved October 19, 2018.
- ^ "Cannabis Legalization and Regulation". Department of Justice, Electronic Communications. Government of Canada. June 20, 2018.
- ^ "'It should be available': Natural health food stores hope to cash in on CBD craze". CBC News. August 6, 2019. Retrieved October 11, 2019.
- ^ Canada Health (June 20, 2018). "Cannabis and Canadian borders". aem . Retrieved September 3, 2020.
- ^ a b c d Chu W (January 31, 2019). "Updated EC ruling for CBD classes supplement ingredient as Novel Food". NutraIngredients.com, William Reed Business Media Ltd. Retrieved January 1, 2019.
- ^ "Cannabinoids, searched in the EU Novel food catalogue (v.1.1)". European Commission. January 1, 2019. Retrieved February 1, 2019.
- ^ "European Commission reverses course, says CBD should not be regulated as a narcotic". Hemp Industry Daily. December 2, 2020. Retrieved December 4, 2020.
- ^ a b "CosIng – Cosmetics – Cannabidiol". European Commission. Retrieved December 4, 2016.
- ^ Fournier G, Beherec O, Bertucelli S (2003). "Intérêt du rapport Δ-9-THC / CBD dans le contrôle des cultures de chanvre industriel" [The advantage of the Δ-9-THC / CBD ratio in the control of industrial hemp crops]. Annales de Toxicologie Analytique (in French). 15 (4): 250–59. doi:10.1051/ata/2003003.
- ^ "Doctors now able to prescribe cannabidiol". radionz.co.nz. June 2, 2017. Retrieved June 2, 2017.
- ^ "CBD products". www.health.govt.nz. Retrieved March 10, 2019.
- ^ "Является ли вещество CBD (каннабидиол) разрешенным к использованию на территории России? - Правовед.RU".
- ^ "Журнал переписки депутата Бесединой".
- ^ "CBD products should follow the drug laws". Swedish Medical Products Agency. April 4, 2018. Retrieved July 31, 2018.
- ^ Arnold M (July 30, 2019). "Sweden Joins Italy In Path To Defining CBD Oil Regulations". Cannabis Industry Journal . Retrieved September 3, 2020.
- ^ "Cannabis: What is allowed, what is not allowed in Switzerland? - www.ch.ch". www.ch.ch . Retrieved September 3, 2020.
- ^ Kohler S, Benz AS, Pruschy D, et al. (Vischer AG) (April 11, 2019). "Trends in the Swiss Cannabis Regulation | Lexology". www.lexology.com . Retrieved September 3, 2020.
- ^ "Cannabis à faible teneur en THC et CBD" (in French). BAG.Admin.ch. Archived from the original on March 26, 2017. Retrieved May 20, 2017.
- ^ Поперечна, Дарія (April 9, 2021). "В Україні легалізували використання медичного канабісу, але не всього". УП.Життя (in Ukrainian).
- ^ "Sativex Oromucosal Spray – Summary of Product Characteristics (SPC)". Medicines.org.uk (eMC). Retrieved December 4, 2016.
- ^ "MHRA statement on products containing Cannabidiol (CBD)". Gov.uk. December 14, 2016.
- ^ "UK Classifies CBD Oil as a Medicinal Ingredient". BuyCBD.net . Retrieved September 3, 2020.
- ^ Stallworth J. "Drug licensing factsheet: cannabis, CBD and other cannabinoids". The Home Office . Retrieved December 10, 2020.
- ^ Gunn L, Haigh L (January 29, 2019). "British watchdog deems CBD a novel food, seeks to curtail sale on UK market". Nutrition Insight, CNS Media BV. Archived from the original on February 2, 2019. Retrieved January 1, 2019.
- ^ a b "Cannabidiol (CBD) guidance - Business guidance on cannabidiol (CBD) as a novel food". UK Food Standards Agency. September 24, 2020. Retrieved December 8, 2020.
- ^ Marsat N (July 7, 2020). "CBD and Novel Foods regulation in the UK: regulation then and now". Health Europa . Retrieved December 10, 2020.
- ^ "International Drug Scheduling; Convention on Psychotropic Substances; Single Convention on Narcotic Drugs; World Health Organization; Scheduling Recommendations; Dronabinol (delta". Federal Register. March 1, 2019. Retrieved November 4, 2019.
- ^ "CANNABIDIOL (CBD) - Critical Review Report | Expert Committee on Drug Dependence 40th Meeting" (PDF). Geneva: World Health Organization. June 7, 2018. Retrieved September 3, 2020.
- ^ Angell T (August 13, 2018). "UN Launches First-Ever Full Review Of Marijuana's Status Under International Law". Marijuana Moment . Retrieved November 1, 2018.
- ^ Mead A (May 2017). "The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law". Epilepsy & Behavior. 70 (Pt B): 288–291. doi:10.1016/j.yebeh.2016.11.021. PMID 28169144.
- ^ Conaway KM (December 20, 2018). "Text - H.R.2 - 115th Congress (2017-2018): Agriculture Improvement Act of 2018". www.congress.gov . Retrieved May 1, 2019.
- ^ a b "What You Need to Know (And What We're Working to Find Out) About Products Containing Cannabis or Cannabis-derived Compounds, Including CBD". US Food and Drug Administration. November 26, 2019. Retrieved November 26, 2019.
- ^ a b Bellamy J (May 9, 2019). "FDA: No CBD in dietary supplements or foods for now, but let's talk". Science-Based Medicine.
- ^ a b c Abernethy A, Schiller L (July 17, 2019). "FDA is Committed to Sound, Science-based Policy on CBD". US Food and Drug Administration (FDA). Retrieved October 17, 2019.
- ^ "DEA reschedules Epidiolex, marijuana-derived drug, paving the way for it to hit the market". CNBC. September 27, 2018.
- ^ "Epilepsy Foundation Statement on DEA's Scheduling of Epidiolex" (Press release). Landover, MD: Epilepsy Foundation. September 27, 2018. Retrieved November 1, 2018.
- ^ "State Rescheduling for FDA-approved Therapies Derived from CBD". Epilepsy Foundation . Retrieved November 1, 2018.
- ^ Maa E, Figi P (June 2014). "The case for medical marijuana in epilepsy". Epilepsia. 55 (6): 783–6. doi:10.1111/epi.12610. PMID 24854149. S2CID 4849160.
- ^ Young S (August 7, 2013). "Marijuana stops child's severe seizures". CNN . Retrieved May 14, 2018.
- ^ a b c "State Medical Marijuana Laws". National Conference of State Legislatures. April 27, 2018. Retrieved May 14, 2018.
- ^ "FDA and Cannabis: Research and Drug Approval Process". US Food and Drug Administration. January 14, 2020. Retrieved March 6, 2020.
- ^ Corba J. "Super Bowl Champ: CBD Can Solve NFL's Opioid Problem'". Cheddar . Retrieved January 29, 2019.
- ^ "FDA and Marijuana: Questions and Answers. No. 12 – Can products that contain THC or cannabidiol (CBD) be sold as dietary supplements?". US Food and Drug Administration (FDA). December 20, 2018. Retrieved January 13, 2019.
- ^ Gaines LV (March 23, 2017). "Why are CBD products sold over the counter some places and tightly regulated in others?". Chicago Reader . Retrieved January 3, 2018.
- ^ Massachusetts says hemp-derived CBD is illegal — but CBD stores are still everywhere
- ^ a b Hahn SM (March 5, 2020). "FDA Advances Work Related to Cannabidiol Products with Focus on Protecting Public Health, Providing Market Clarity". US Food and Drug Administration. Retrieved March 6, 2020.
- ^ a b "Sampling Study of the Current Cannabidiol Marketplace to Determine the Extent That Products are Mislabeled or Adulterated Report in Response to Further Consolidated Appropriations Act, 2020" (PDF). United States Food and Drug Administration. July 2020.
- ^ Bonn-Miller MO, Loflin MJ, Thomas BF, Marcu JP, Hyke T, Vandrey R (November 2017). "Labeling Accuracy of Cannabidiol Extracts Sold Online". JAMA. 318 (17): 1708–1709. doi:10.1001/jama.2017.11909. PMC5818782. PMID 29114823.
- ^ a b MacKeen D (October 16, 2019). "Scam or Not: What Are the Benefits of CBD?". The New York Times . Retrieved October 17, 2019.
- ^ Evans DG (2020). "Medical Fraud, Mislabeling, Contamination: All Common in CBD Products". Missouri Medicine. 117 (5): 394–399. PMC7723146. PMID 33311737.
- ^ "Cannabidiol (CBD)". American Association of Poison Control Centers. September 30, 2019. Retrieved October 17, 2019.
- ^ "The 2014 Farm Bill". thefarmbill.com . Retrieved November 27, 2018.
- ^ Zhang M. "No, CBD Is Not 'Legal in All 50 States'". Forbes . Retrieved November 27, 2018.
- ^ 7 U.S.C. § 1639o
- ^ 21 U.S.C. § 802
- ^ "DEA announces steps necessary to improve access to marijuana research". United States Drug Enforcement Administration. August 26, 2019. Retrieved October 18, 2019.
- ^ "FDA Warns Companies Illegally Selling CBD Products". US Food and Drug Administration. December 22, 2020. Retrieved January 12, 2021.
The warning letters include CBD products that are especially concerning from a public health perspective due to the route of administration, including nasal, ophthalmic and inhalation. In addition, they address violations relating to the addition of CBD to food, and the impermissible marketing of CBD products as dietary supplements.
- ^ "FTC Announces Crackdown on Deceptively Marketed CBD Products". US Federal Trade Commission (FTC). December 17, 2020. Retrieved January 12, 2021.
- ^ a b Ashley DD, Engle MK (October 22, 2019). "Warning letter: Rooted Apothecary LLC". Office of Compliance, Center for Drug Evaluation and Research, US Food and Drug Administration; US Federal Trade Commission. Retrieved October 23, 2019.
Further reading [edit]
- Williams A (October 27, 2018). "Why Is CBD Everywhere?". The New York Times. ISSN 0362-4331.
External links [edit]
- "Cannabidiol". Drug Information Portal. U.S. National Library of Medicine.
What Does Kj Stand for in Food
Source: https://en.wikipedia.org/wiki/Cannabidiol
0 Response to "What Does Kj Stand for in Food"
Post a Comment